Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Studies on Extraction and Biomedical Application of Marine Collagen
*Corresponding author:Du Juan, Zhuhai College of Science and Technology, Zhuhai, China.
Received: March 19, 2024; Published: March 22, 2024
DOI: 10.34297/AJBSR.2024.21.002904
Abstract
The ocean is rich in biological resources and active ingredients. Among them, marine collagen can be used as a new type of biomaterial in scientific research, medical and cosmetic industries due to its characteristics of good water solubility, high compatibility and environmental protection. In addition to the edible part of fish, fish skin, fish bones, fish scales can be extracted collagen in biomedicine has been widely used. Marine collagen is a multifunctional compound that can influence the process of wound healing. Collagen is enzymatically cleaved to form collagen peptide, which is a small molecule active peptide that can be directly absorbed by the human body, and it has been shown that it can be used in the treatment of obesity, atopic dermatitis and melanin growth. In this paper, marine collagen and its application in biomedicine are discussed, and the collagen from drab filefish skin was enzymatically extracted and made into edible film, and the edible film was made by adding additives EGCG (tea polyphenol) and CS (chitosan), and the optimal formula for the edible film based on the collagen from drab filefish skin was 1.0g of fish collagen, 0.1g of EGCG and 0.01g of CS, 10ml of 0.5mol/L acetic acid. Drying at 70°C for 1h was found to be the optimum film formation temperature. The results provide a theoretical basis for the extraction and application of marine collagen.
Keywords: Drab filefish Skin, Collagen, EGCG, CS, Collagen film
Introduction
According to the relevant data of 2022 China Fishery Statistical Yearbook, in 2022, the total production of aquatic products in China reached 68,659,100 tonnes, and the total amount of its processing reached 21,477,900 tonnes, the fishery and aquaculture production reached a new record high, and aquatic food has made an important contribution to ensuring food security and nutrition in the 21st century. Fish is processed in large quantities, and the by-products of fish processing include fish skin, fish scales, fish bones, fish offal and so on. According to statistics, the by-products of processed aquatic products can reach 40 to 50 per cent of the total production [1]. In the past, fish skin, fish scales, fish bones, etc. were considered to have low added value, and most of them were still used as feed or directly discarded, and how to reuse them is the key to the use of marine biological resources. Aquatic collagen is an excellent biological material, widely used in medicine, cosmetics, food processing and other fields, the largest proportion of freshwater fish processing by-products [2]. Aquatic collagen is mainly produced from fish processing by-products such as fish skin, bones, cartilage and scales, which has the advantages of good biocompatibility, no risk of animal diseases and pathogens, and low content of biological contaminants and toxins, and has attracted considerable attention from researchers and scientists in recent years [3].
Collagen is a structural tissue protein and is a major protein component of the extracellular matrix and connective tissues of animals. Collagen is relatively abundant in mammals and is mainly found in the extracellular matrix (ECM) of fibrous connective tissues, where it has properties such as ductility and flexibility [4]. Collagen is a protofibre and the connective tissue is rich in different types of collagen, which are classified as types I, II, III, V and XI [5]. Type I collagen is the most common and abundant type of collagen found in mammalian tendons and skin. Collagen peptides are transparent collagen extracts consisting of two or more amino acids, which are small peptides formed when collagen is processed by artificial proteases. Compared to collagen, collagen peptides have a lower molecular weight and are more easily absorbed and utilised by the body. Although there are differences between collagen and collagen peptides, they are closely related. Collagen is the parent protein of collagen peptides, while collagen peptides are low molecular weight fragments produced after collagen is biologically processed [6-8]. Fish collagen and terrestrial animal collagen amino acid structure is similar, with good water solubility, biosafety, biodegradability, etc, and its living environment is relatively clean, in the control of viruses, cross-infection and other risks than the terrestrial animals have obvious advantages and is considered to be an important source of medical collagen replacement [9,10].
Fish Skin Collagen Extraction Method
Collagen is a biological macromolecule and the main component of animal connective tissue. It is also the most abundant and widely distributed functional protein in mammals [11]. Due to differences in amino acid composition and cross-linking degree, collagen derived from aquatic animals, particularly from processing wastes such as skin, bone, and scales, offers several advantages over livestock collagen. Collagen from marine animals is superior to terrestrial animal collagen in certain aspects, such as fish skin. It is important to note that this is an objective evaluation based on scientific evidence and not a subjective opinion. Collagen is widely used in various fields such as food, medicine, tissue engineering, and cosmetics due to its low antigenicity, hypoallergenicity, good biocompatibility, biodegradability, and biological activity [12,13].
The extraction of collagen from fish skin involves changing the external environment (e.g. pH, salt concentration, temperature) in which the protein is located using physicochemical and other methods that are specific to the characteristics of collagen. This allows the collagen to separate from the raw material. The main extraction methods currently used are heat extraction, acid extraction, alkali extraction, enzyme extraction, salt extraction, and ultrasound-assisted extraction.
Hot Water Extraction Method
The hot water extraction method is based on the principle of pre-treating raw materials and then treating them with hot water to greatly improve their solubility properties. As a result, the general collagen extraction rate increases with temperature. However, high temperatures can lead to the destruction of the superhelical structure of collagen, which means that the collagen extracted by this method, also known as gelatin, does not have the physiological function of collagen [14]. Lin Wang and colleagues extracted gelatin from the swim bladder of Acipenser schrenckii (Amur sturgeon) using a hot water extraction method at 50°C. The extraction rate was 76.54%, and the extracted product exhibited characteristics of type I collagen [15].
Acid Extraction Method
The acid extraction method is based on the principle of using an acidic environment to disrupt and denature the specific chemical structure of the collagen molecule, allowing for its separation from the raw material. The effect of an acidic environment on collagen molecules results in hydrolysis and denaturation of collagen. In their study, Samaneh Pezeshk, et al. extracted collagen from tuna fish skin using an acid extraction method. They used a concentration of 0.5mol/L acetic acid, a liquid to material ratio of 1:30, and extracted for 48 hours at a temperature of 4°C. The final yield of collagen obtained was 5.47% dry weight and 21.49% wet weight [16].
Alkali Extraction Method
Collagen extraction involves the use of a specific concentration of alkali under certain external conditions. Lime, sodium hydroxide, and sodium carbonate are commonly used as treatment agents. In experiments, a combination of acid and alkali is typically used to extract type I collagen polypeptides. Wen Huifang, et al. extracted collagen from catfish skin using an alkali extraction method. The process conditions involved a material-liquid ratio of 1:10, the addition of 1.6% Ca (OH)2, and heating at 70℃ for 9 hours. The resulting collagen extraction rate was 79.67%. The extracted collagen is typical of type I collagen peptides, which is consistent with the method used by most researchers [17].
Ultrasonic-Assisted Extraction Method
Ultrasound-assisted extraction (UAE) is a physical method that utilizes ultrasound to break down complex molecules into smaller compounds. This method is considered to be more environmentally friendly than chemical methods [18]. Ultrasonication can be used to disrupt the tissue structure of fish skin, exposing collagen. When combined with enzymatic digestion, this method can increase the rate of collagen extraction, shorten the extraction time, and improve overall efficiency. Bao, et al. prepared fish skin by thawing and weighing it at room temperature under running water. They then soaked it in sodium hydroxide to remove heterogeneous proteins and washed it under running water until the pH value was neutral. To remove the fat, the skin was soaked in n-butanol and washed under water before being immersed in acetic acid for ultrasonication. The results indicated that: The collagen extraction rate reached 68.67%, which was the highest extraction rate achieved with an ultrasonication power of 310 W, ultrasonication time of 10 minutes, and enzymatic degradation time of 29 hours [19].
Enzymatic Digestive Method
Enzymatic extraction of collagen aims to use different proteases to restrict the degradation of collagen under suitable conditions, which can be soluble in low concentrations of organic acids or neutral solutions and thus be extracted, common enzymes are pepsin, protease, trypsin and so on [20]. Zhao, et al. extracted cod skin collagen enzymatically, and the collagen yield was 21.92% when the pepsin addition was 1% and the material-liquid ratio was 1:10 [21].
Enzymatic Extraction was Used to Extract Collagen from the Skin of Horse Mackerel
Experimental Instruments
The main pharmaceutical materials used in this experiment were acetic acid, sodium chloride, sodium hypochlorite, calcium chloride, pepsin, CS (Chitosan), EGCG (Polyphenol), drab filefish, etc. The main experimental instruments include LGJ-10 Freeze Dryer, HH Digital Display Constant Temperature Water Bath, FA22048 Electronic Balance, 5804R Refrigerated Centrifuge, HY-3 Multi-purpose Oscillator, and Ultraviolet Spectrophotometer, Ultrasound Machine, Magnetic Stirrer, etc.
Experimental Methods
Extraction of fish skin collagen (Note: The extraction process was carried out at 4°C to prevent collagen denaturation.)
Raw material pre-processing: Prepare the fresh fish skin by washing and drying it, then cutting it into small pieces measuring 0.5cmx5cm. Store the pieces at -20℃ and thaw them at 4℃ when ready to use.
Decolourisation and protein removal: The fish skin was soaked in a 0.1mol/L NaOH solution containing 10% sodium hypochlorite and 6% NaCl in a 1:10 ratio for 1h.
Swell: The fish skin after decolourisation and removal of heterogeneous proteins was washed to neutrality with deionised water, added with 1:5 buffer and solubilised for 1h.
Enzymolysis: Acid hydrolase was used at an enzyme level of 1000u/g, a feed-to-liquid ratio of 1:5, a pH value of 3.0, and an enzyme digestion time of 1h. Under these conditions, the extraction rate of collagen could reach 57.95%.
Salting out: The supernatant was taken after centrifugation of the enzyme solution, and solid NaCl was added while stirring until the concentration of NaCl reached 2.5mol/L, and it was completely dissolved and then left for 30min. then 10,000r/min freezing centrifugation was performed for 10 min, and the precipitate was collected.
Dialysis (separation of crystalloids by osmosis): The precipitate was redissolved in 0.5mol/L acetic acid and dialysed with 0.1mol/L acetic acid for 1h, followed by deionised water for 1h, in which the extradialysis solution was changed every 20min.
Freeze drying: The dialysate was freeze-dried to obtain fish skin collagen.
Collagen Peptide Uses
Fish Skin Collagen Peptides for Obesity
Obesity refers to the disorder of energy balance caused by long-term energy intake exceeding energy expenditure, resulting in collective excess energy storage in the form of fat. Collagen peptides can play a role in improving obesity by regulating intestinal flora, regulating lipid metabolism, and inhibiting adipose differentiation. Ga Hyeon Baek, et al. found that collagen can change the composition of gut microbes, by reducing the proportion of gut Firmicutes and Bacteroidetes and increasing the abundance of specific bacterial taxa, Such as Bacteroides, Streptococcus, Faecalibaculum, and Clostridiumsensu stricto1 to exert anti-obesity effects [22]. Furthermore, ingestion of fish skin collagen caused significant changes in gut microbiota, demonstrating its potential as a therapeutic agent for obesity [23].
Effect of Fish Collagen Peptides on Melanin Growth
Melanin is a biological pigment that is formed by a series of chemical reactions of tyrosine or 3, 4-dihydroxyphenylalanine. Melanin is an amino acid derivative found in the cells of the basal layer of the skin. In addition, melanin protects skin cells from ultraviolet (UV) damage. However, excessive expression of melanin, which moves layer by layer through cell metabolism and reaches the surface of the skin, can lead to dark complexion, pigmentation, and other problems.
Hai-Lan Li, et al. showed that silver carp scale collagen peptide-1 (SCPs-1) could inhibit melanin formation by increasing GSH content and reducing tyrosinase activity, ROS and cAMP content [24].
Fish Skin Collagen Peptides for the Treatment of Atopic Dermatitis
Atopic dermatitis (AD), also known as atopic dermatitis, atopic eczema, Besnier's constitution prurigo, or genetic allergic eczema, is a kind of allergic skin disease with eczema-like changes of the skin related to genetic allergic constitution. It is often recurrent in children and young adults. The study by Amiko Hakuta, et al. confirmed that the pathway of collagen peptides in the treatment of AD was achieved by inhibiting the mRNA and protein expression of TARC and TSLP channel signaling factors [25].
Fish Skin Collagen for Wound Healing
The skin's epidermis is the largest organ in the human body and serves as the primary innate defence barrier against pathogens [26]. Marine collagen is extracted from the soft and hard connective tissues of Marine organisms. After chemical and enzymatic hydrolysis, the small molecular weight of Marine collagen makes it more water-soluble, which also makes Marine collagen peptides have greater advantages in the treatment of skin epidermal wounds [27-29].
Cao, et al. found a human-like collagen-carboxymethylated chitosan (HRC-CCS) skin scaffold water film formation solution to promote the regeneration of wound skin tissue [30]. Pan, et al. used collagen to synthesize a multifunctional PVA/HRC/SA composite aqueous film solution with gas permeability, bacterial barrier, and hemostatic activity, which can promote wound healing of full-thickness skin [31].
Preparation of Collagen Membranes from Drab Filefish Skin
The horse-faced fish is found in four major sea regions of our country, with an annual output of over 250,000 tons in the East China Sea alone, making it the second most important marine economic fish in our country. As consumers become more environmentally conscious and demand safer food, there is a growing interest in non-toxic and harmless green packaging materials, such as edible films made of polysaccharides, proteins, starch, cellulose, and more. One such material is the edible collagen film made of collagen, which provides excellent physical properties, and good biocompatibility, and improves the sensory performance of the food. It is also easy to produce, degrades naturally, and is pollution-free. Unfortunately, due to the thick and hard skin of the horse-faced fish, which accounts for 8.5% to 9.4% of the fish's weight, the skin is usually discarded after fishing while the meat is refrigerated for sale. This leads to significant resource waste and environmental pollution.
The study of the edible collagen membrane of horse flounder skin found that a single edible collagen membrane of horse flounder skin cannot provide high mechanical strength. To strengthen its performance, this study adopted physical or chemical treatment methods to promote crosslinking between protein molecules and modify the polymer network to improve the performance of the edible collagen membrane of horse flounder skin. In addition, the addition of EGCG (polyphenol) and CS (chitosan) in two or more polymer blend films has been achieved to improve the physical and chemical properties of the film. Adding polysaccharides to the protein matrix can enhance the performance of the film. Among them, chitosan (CS) is widely used, which has good biocompatibility and biodegradability, high hydrophilicity and bio adhesiveness. In addition, polyphenol (EGCG), a phenolic compound, has antioxidant activity and bacteriostasis; polyphenol can improve chitosan's emulsifying activity and antioxidant activity.
This study utilized collagen from horse face fish skin to form a matrix for membrane preparation. Chitosan (CS) and polyphenols (EGCG) were added to create a composite membrane. The best formula for forming the composite membrane was determined to provide a theoretical framework for the development of new edible packaging films.
Preparation of an edible collagen membrane from drab filefish skin
The film solution was prepared by dissolving 1.0g fish skin collagen in 10mL 0.5mol/L acetic acid, adding the appropriate amount of CS and EGCG, and stirring with a magnetic stirrer for 30minutes. After it was completely dissolved, the bubbles were removed by ultrasound, and the film was dried on an organic glass plate at 70℃ for 1h by flow casting method.
Results
To avoid chance, all experimental groups were repeated three times, and the mean value was selected to record the experimental phenomena.
Investigating the Effect of Collagen and Chitosan Dosage on Film-Forming Effects
Weigh 0.5g, 0.8g, 1.0g, 1.2g of fish skin collagen, respectively, add 0.1g of EGCG (polyphenols) and 0.001g and 0.01g of CS (chitosan) to which 10ml of 0.5mol/L acetic acid was added to mix thoroughly, stirred with a magnetic stirrer for 30min, and when it was completely dissolved, ultrasonic to remove bubbles, and then placed on a Plexiglas plate for 1h at 70°C using the flow-through method. The film was dried on a plate at 70°C for 1h, and the film was taken after drying.
Through the first 4 groups of experiments in Table 1, when the content of chitosan is 0.001g, the experimental phenomena are manifested as the film-forming solution and the glassware are too sticky and dense and fail to form a film. From the film-forming mechanism of fish skin collagen and chitosan, it can be analyzed that: as chitosan is connected by intramolecular and intermolecular hydrogen bonding to form a viscous film-forming solution, during the drying process of film-forming solution, water evaporation, intermolecular hydrophobic interactions between the CS molecules and the degree of entanglement increased, and it can be inferred that the content of chitosan is too small, resulting in the failure to form a film. Therefore, in the last four sets of experiments, after increasing the amount of chitosan, it can be found that the film-forming solution was more viscous, and a film was formed in some.areas on the surface. Therefore, the amounts of fish skin collagen and chitosan were 1.0 g and 0.01 g, respectively.
Exploring the Effect of Drying Temperature on Film-Forming Effect
Weigh 1.0g of fish skin collagen, add 0.01g of EGCG (polyphenols) and 0.01g of CS (chitosan) to which add 10ml of 0.5mol/L acetic acid to mix thoroughly, stirred with a magnetic stirrer for 30min, to be completely dissolved, ultrasound to remove air bubbles, using the method of casting on a plexiglass plate were at 65℃, 70℃, 75℃, 80℃. Dry for 1h, take the film after drying.
From the experimental data in Table 2, it can be seen that under the same experimental conditions, when the drying temperature is not enough, the water in the film-forming solution is not dried, and the degree of hydrophobic interactions and entanglement between CS molecules is not enough to form a film. Increasing the drying temperature can make the hydrophobic amino acid side-chain residues originally hidden inside the collagen molecules exposed on the molecular surface, and at the same time, it is also conducive to the interaction between collagen molecules and chitosan molecules, and many hydrogen bonds are formed within the molecules and between the molecules, which promotes the formation of the membrane. However, when the drying temperature is too high, it will not only cause the denaturation of fish skin collagen in the film-forming solution, but also provide an extra share of energy to destroy the intermolecular hydrogen bonding, thus affecting the effect of film formation. Therefore, the drying temperature is 70°C.
Investigating the Impact of Drying Time on the film-Making Process
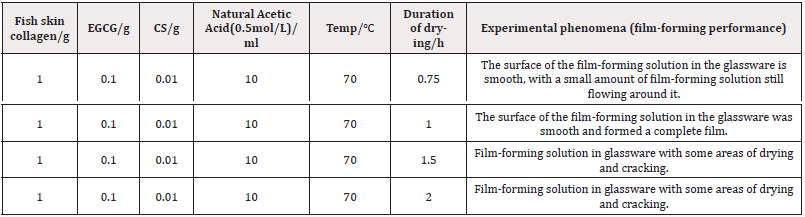
Weigh 1.0g of fish skin collagen, add 0.01g of EGCG (polyphenols) and 0.01g of CS (chitosan) to which add 10ml of 0.5mol/L acetic acid to mix thoroughly, stirred with a magnetic stirrer for 30min, to be completely dissolved, ultrasound to remove air bubbles, using the method of flow-through placed on a plexiglass plate at 70℃ were dried for 0.75h, 1h, respectively, 1.5h, 2h, take the film after drying.
As can be seen from the experimental data in Table 3, under the same experimental conditions, the drying time is too short, the water in the film-forming solution is not completely dried, and the degree of hydrophobic interactions and entanglement between CS molecules is not enough to form a film. Extending the drying time too long helps to increase the probability of interaction between collagen and chitosan and promotes film formation. However, the drying time was too long, and the water in the film-forming solution was completely evaporated, making the film-forming solution stick to the glassware or even dry cracking in some areas. Therefore, the drying time was 1h.
Conclusion
This paper reviews different methods for extracting marine collagen from fish, including hot water extraction, acid extraction, alkali extraction, and ultrasound-assisted extraction. The paper focuses on the enzymatic extraction of collagen from horse mackerel skin. Furthermore, this article summarises the biomedical applications of collagen and collagen peptides in wound healing, obesity, melanin growth, anti-specific dermatitis, and the preparation of edible horse mackerel skin collagen membranes. The aim of this study was to investigate the optimal criteria for forming collagen film from horse mackerel skin. Various factors, such as horse mackerel skin collagen, chitosan, baking temperature, drying time, and glycerol content were examined. The study found that the optimal conditions for forming collagen film were dependent on the values of these factors. At the end of the experiment, we discovered the simple and efficient process and conditions for extracting collagen from horse mackerel skin. We also determined the optimal ratio for preparing horse mackerel skin collagen films. These findings provide valuable insights for the production of collagen film products. Collagen is a bioactive substance with great market potential. Collagen peptides have been shown to improve blood lipid levels, making them effective in anti-obesity treatments. Additionally, collagen peptides have been found to improve atopic dermatitis. These findings provide new ideas for the development of natural health drugs. Therefore, it is important to continue researching and discovering marine collagen sources and applications. The benefits and potential for future biomedical applications are vast and should not be overlooked.
Acknowledgment
Fund Project
1. Guangdong College Students’ Innovation and Entrepreneurship Training Program in 2022(DC2023083).
2. Guangdong University Characteristic Innovation Program (Natural Science) Fund (2021KTSCX173).
References
- Gao Rui Chang ZLL, Zhang Wei, Wang Lin, Yuan Li (2023) High value utilization and research prospect of processing by-products derived from freshwater fish. Food Safety and Quality Detection Technology 14(13): 203-210.
- Ahmed M, Verma AK, Patel R (2020) Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustainable Chemistry and Pharmacy 18: 100315.
- Prajaputra V, Nadia Isnaini, Siti Maryam, Ernawati Ernawati, Fitri Deliana, et al. (2024) Exploring marine collagen: Sustainable sourcing, extraction methods, and cosmetic applications. South African Journal of Chemical Engineering 47: 197-211.
- Whelan A, J Duffy, RT Gaul, DO Reilly, DR Nolan, et al. (2019) Collagen fibre orientation and dispersion govern ultimate tensile strength, stiffness and the fatigue performance of bovine pericardium. J Mech Behav Biomed Mater 90: 54-60.
- Holmes DF, Lu Y, Starborg T, Kadler KE (2018) Collagen Fibril Assembly and Function. Curr Top Dev Biol 130: 107-142.
- Nicol L, Patrick Morar, Ying Wang, Kim Henriksen, Shu Sun, et al. (2019) Alterations in non-type I collagen biomarkers in osteogenesis imperfecta. Bone 120: 70-74.
- Chowdhury SR, Mohd Fauzi Mh Busra, Yogeswaran Lokanathan, Min Hwei Ng, Jia Xian Law, et al. (2018) Collagen Type I: A Versatile Biomaterial. Adv Exp Med Biol 1077: 389-414.
- Kisling A, Lust RM, & Katwa LC (2019) What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci 228: 30-34.
- Furtado M, Chen L, Chen Z, Chen A, Cui W (2022) Development of fish collagen in tissue regeneration and drug delivery. Engineered Regeneration 3(3): 217-231.
- Jafari H, Alberto Lista, Manuela Mafosso Siekapen, Pejman Ghaffari Bohlouli, Lei Nie, et al. (2020) Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 12(10): 2230.
- Shenoy M, Nishath Sayed Abdul, Zeeshan Qamar, Bader Musfer Al Bahri, Khalid ZK Al Ghalayini, et al. (2022) Collagen Structure, Synthesis, and Its Applications: A Systematic Review. Cureus 14(5): e24856.
- Gauza Włodarczyk M, Kubisz L, Włodarczyk D (2017) Amino acid composition in determination of collagen origin and assessment of physical factors effects. International Journal of Biological Macromolecules 104: 987-991.
- Li Y, Yuzhe Liu, Ronghang Li, Haotian Bai, Zhengqing Zhu, et al. (2021) Collagen-based biomaterials for bone tissue engineering. Materials & Design 210: 110049.
- Derkach S, Kolotova D, Kuchina Y, Shumskaya N (2022) Characterization of Fish Gelatin Obtained from Atlantic Cod Skin Using Enzymatic Treatment. Polymers 14(4).
- Wang L, Xiaoxiao Wang, Fan Bai, Yong Fang, Jinlin Wang, et al. (2019) The anti-skin-aging effect of oral administration of gelatin from the swim bladder of Amur sturgeon (Acipenser schrenckii). Food & function 10(7): 3890-3897.
- Pezeshk S, Rezaei M, Abdollahi M (2022) Impact of ultrasound on extractability of native collagen from tuna by-product and its ultrastructure and physicochemical attributes. Ultrasonics Sonochemistry 89: 106129.
- Wen Hui fang ZL, Chen Li li, Yuan Mei lan, Bai Chun qing (2015) Study on process optimization of extracting collagen from Amiurus nebulosus skin by alkali way. Science and Technology of Food Industry (19): 233-236.
- Noor NQIM, Rina Syafinaz Razali, Nur Khairina Ismail, Rabiatul Amirah Ramli, Umi Hartina Mohamad Razali, et al. (2021) Application of Green Technology in Gelatin Extraction: A Review.
- Wang Jin mei, Bao Jian qiang (2017) The ultrasonic-assisted solvent extraction and characterization of fishskin collagen. Acta Agriculturae Shanghai 33(2): 114-119.
- Laasri I, Bakkali M, Mejias L, Laglaoui A (2023) Marine collagen: Unveiling the blue resource-extraction techniques and multifaceted applications. Int J Biol Macromol 253: 127253.
- Liu Shan, Liu Peiyong, Liu Liangzhong, Li Xiaona, Tan Beini (2013) Study on decolorization process of sturgeon skin collagen peptide hydrolysate[J]. Food Industry 34(9): 120-123.
- Baek GH, Ki Myeong Yoo, Seon Yeong Kim, Da Hee Lee, Hayoung Chung, et al. (2023) Collagen Peptide Exerts an Anti-Obesity Effect by Influencing the Firmicutes/Bacteroidetes Ratio in the Gut. Nutrients 15(11): 2610.
- Lee EJ, Jinwoo Hur, Sun Ah Ham, Yeonji Jo, SangYoon Lee, et al. (2017) Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int j biol macromol 104(Pt A): 281-286.
- Möller JKS, Kinga Linowiecka, Maciej Gagat, Anna A Brożyna, Marek Foksiński, et al. (2023) Melanogenesis Is Directly Affected by Metabolites of Melatonin in Human Melanoma Cells. Int j mol sci 24(19): 14947.
- Hakuta A, Yukie Yamaguchi, Tomoko Okawa, Shoko Yamamoto, Yasuo Sakai, et al. (2017) Anti-inflammatory effect of collagen tripeptide in atopic dermatitis. J dermatol sci 88(3): 357-364.
- Blanpain C & Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. N rev Mol cell biol 10(3): 207-217.
- Chattopadhyay S & Raines RT (2014) Review collagen-based biomaterials for wound healing. Biopolymers 101(8): 821-833.
- Hu Z, Yang P, Zhou C, Li S, Hong P (2017) Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar Drugs 15(4): 102.
- LI Ying Qi, Gong Jin Song, XU Zheng Hong, Shi Jin Song (2020) Recombinant expression and fermentation of type Ⅲ human-like collagen in Escherichia coli. J Microbiol China 47(12): 4154-4171.
- Cao J, Wang P, Liu Y, Zhu C, Fan D (2020) Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. Int J Biol Macromol 155: 625-635.
- Pan H, Daidi Fan, Zhiguang Duan, Chenhui Zhu, Rongzhan Fu, et al. (2019) Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater sci eng C Mater for biol appl 105: 110118.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.